General Surgeons
General surgeons can improve chronic wound care protocols and patient recovery times with this product by enhancing blood flow and accelerating healing.

DermaBind is a premier biologic skin substitute derived from placental membranes to treat chronic wounds.
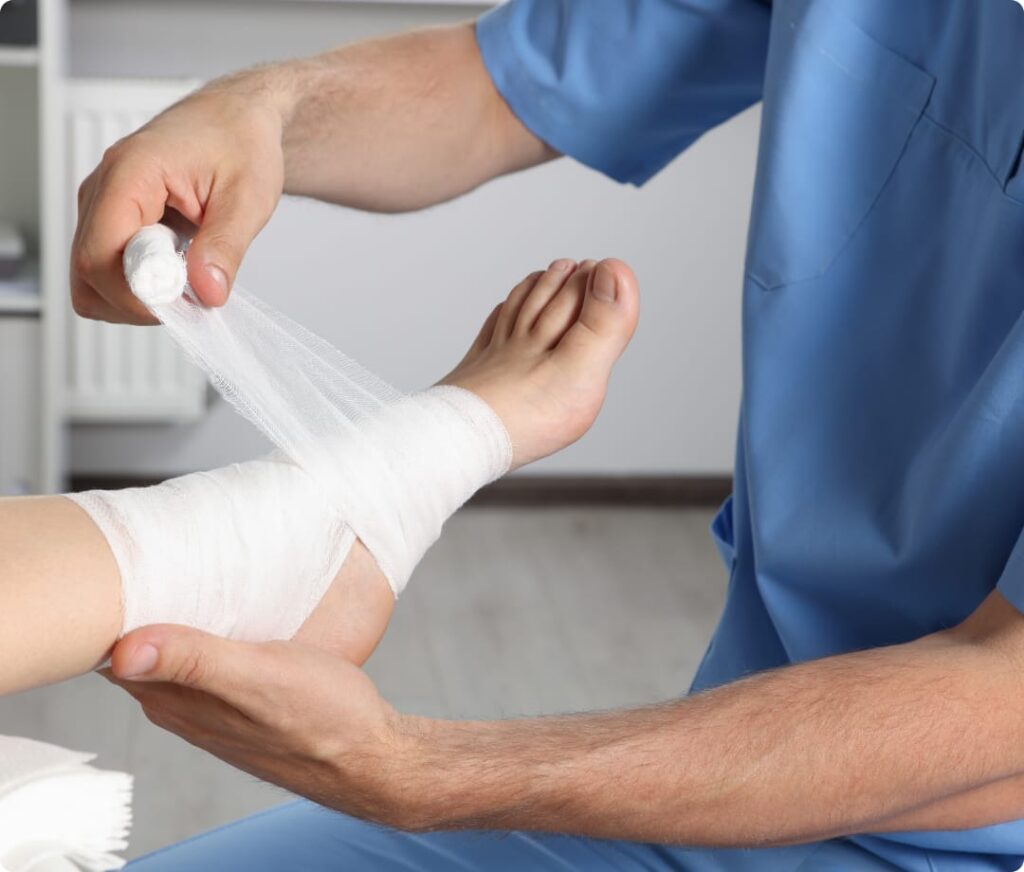
DermaBind is a biologic skin substitute derived from an intact placental membrane. This true three-layer allograft is applied after a 30-day period if the wound has not healed with basic treatments, providing an advanced option for difficult-to-heal wounds. This biologic graft works by increasing blood flow, removing dead tissue, and promoting healing from the bottom up, offering a comprehensive solution for stalled wound healing.
To ensure appropriate use, documented medical necessity and wound dimensions are required for insurance/billing pre-approval.
Whether you need to buy wound care products for diabetic ulcers, pressure ulcers, or other chronic wounds, this comprehensive solution can help.
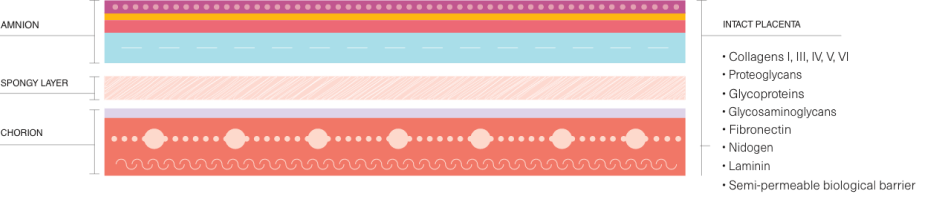
DermaBind undergoes a proprietary preservation method, which retains all native layers of the placental membrane, including the amnion and chorion, with the spongy layer intact, retaining necessary growth factors and cytokines.
This skin substitute contains 400+ proteins, including collagens, fibrins, elastins, glycosaminoglycans, and glycoconjugates. The manufacturing process does not use unnecessary chemicals, antibiotics, or preservatives and is never frozen.

DermaBind is the most advanced treatment option for chronic, or stalled wounds, such as diabetic foot ulcers (DFU), venous leg ulcers (VLU), pressure injuries, and dehisced wounds.
Clinical
This product is one of the thickest allografts on the market, packed with growth factors that reduce the number of applications needed to close a wound to full closure, enhancing clinical outcomes and patient satisfaction.
Billing/Reimbursement
Support will be provided to expedite pre-authorization, appeals, and denials through the manufacturer’s secure online portal to ensure the claim will be covered by Medicare/Medicare Advantage Plans.
Guidance on submitting notes for reimbursement is available in partnership with the manufacturer’s clinical staff to ensure timely reimbursement.
Financial
We can match you to a program that works for your practice with no upfront costs, using our high reimbursement products. These programs are true partnerships that lead to success when working with the manufacturer. Our goal is to give you peace of mind when using skin substitutes and ensure a positive experience for you and your patients.
Schedule a meeting to learn more about DermaBind and how it can impact your office.
By submitting this form, you agree to be contacted by Global Supply Exchange representatives regarding your inquiry. We value your privacy and will not share your information with third parties.
Submitting this form does not obligate you to purchase, but it allows us to provide you with the information and assistance you need.
Schedule a meeting to learn more about DermaBind and how it can impact your office.
By submitting this form, you agree to be contacted by Global Supply Exchange representatives regarding your inquiry. We value your privacy and will not share your information with third parties.
Submitting this form does not obligate you to purchase, but it allows us to provide you with the information and assistance you need.
Various businesses and organizations reap significant benefits from purchasing DermaBind.

General surgeons can improve chronic wound care protocols and patient recovery times with this product by enhancing blood flow and accelerating healing.

Podiatrists can use a biologic skin substitute to treat leg and foot ulcers, improving blood flow and patient outcomes.

Vascular surgeons benefit from DermaBind for treating wounds caused by poor circulation, enhancing blood flow and comprehensive healing.

Diabetic patients see improved care with this solution, which speeds up the healing of ulcers and reduces infection and amputation risks.

Bedridden patients with pressure ulcers experience faster healing, and better care enhanced blood flow and less dead tissue.

Hospitals enhance patient care and efficiency by using this wrap for chronic or difficult-to-heal wounds, offering advanced treatment options.
The manufacturing of DermaBind begins with using human placenta membranes, which naturally protect the fetus during pregnancy. These membranes provide elasticity and strength, prevent maternal and fetal blood mixing, and facilitate nutrient transfer. The proteins and collagen matrix enhance the body’s self-repair capabilities and support effective wound healing.
This product is unique due to its superior manufacturing process, which preserves the integrity of all layers of the placenta membrane, including the amnion and chorion. This meticulous preservation ensures exceptional quality compared to other placental allografts.
Quality Assurance
This product is produced following cGMP, cGTP, and ISO 13485 standards, using placental tissues from donors with normal, full-term pregnancies.
Superior Cleaning Process
We avoid antibiotics and harsh chemicals, employing an aseptic cleaning process that eliminates the need for chemical removal.
Drying Process
An evaporative dryer maintains the membrane’s structure, giving the skin substitute a three-year shelf life.
Superior Radiation Process
Our radiation process is gentler, using 17.5 kilogray compared to the 25 kilogray commonly used, preserving the protein structure and ensuring the biomaterial’s efficacy.
We offer two effective reimbursement options through Medicare/Medicare Advantage plans:
Contact our team for detailed information on reimbursements and current rates. We are here to assist you in navigating the reimbursement process efficiently.
DermaBind is suitable for chronic wounds, diabetic foot ulcers, venous leg ulcers, and pressure ulcers where traditional wound care products have failed.
Visit our commercial website for traditionally treated dressings.
DermaBind is available in four sizes:
DermaBind should be maintained at ambient room temperature before application and should not be frozen. It can be stored for up to three years at room temperature.
The biologic skin substitute is shipped directly from the manufacturer in Salt Lake City, Utah. It is commonly shipped via second-day delivery, with an overnight delivery option available.
This product has a 3-year shelf life at ambient temperatures.
| Resource | Description |
|---|---|
| DermaBind Product Overview | This is a product overview that includes the manufacturing process, intended use, product specifications, and application. |
| DermaBind-TL Fact Sheet | This sheet provides a summary of DermaBind-TL product. |
| DermaBind-FM Fact Sheet | This sheet provides a summary of DermaBind-FM product. |
| Provider Onboarding Packet | The World Reach Health onboarding packet includes a new Customer Form, Fulfillment Form, and product pricing schedule. |
| Insurance Verification Form (IVR) | The IVR Form is sent to Health Tech for pre-authorizations. |
| DermaBind Order Form | The order form includes pricing. |
| Reimbursement Guide | This guide outlines guidance for reimbursement/billing including HCPCS CPT and Q codes. |
Whether you need to buy wound care products for diabetic ulcers, pressure ulcers, or other chronic wounds, this comprehensive solution can help.

P : (407) 813-2442
E : sales@globalsupplyexchange.com
© 2025 Global Supply Exchange
| Powered by Sertain Creative