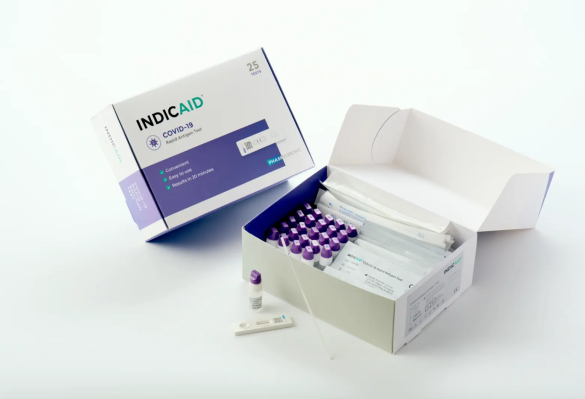
Description
The Phase Scientific INDICAID Point of Care (CLIA WAIVED) Rapid Antigen Test is a reliable, rapid diagnostic tool designed to detect SARS-CoV-2, the virus responsible for COVID-19. This test is ideal for healthcare professionals who need quick and accurate results in various settings, including clinics, hospitals, and urgent care centers. With a simple procedure and easy-to-read results, the INDICAID test offers an efficient solution for COVID-19 testing, helping to identify infections early and prevent further spread of the virus.
What is the Phase Scientific INDICAID Point of Care Rapid Antigen Test?
The Phase Scientific INDICAID Point of Care Rapid Antigen Test is a lateral flow immunoassay designed for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2. This test is intended for use by healthcare professionals in point-of-care settings to quickly identify individuals who may be infected with COVID-19. The test provides results in approximately 20 minutes, making it a valuable tool for screening in situations where time is critical. Its ease of use and rapid turnaround make it a trusted choice for healthcare providers.
Phase Scientific INDICAID COVID-19 POC Rapid Antigen Test Key Features
The INDICAID Point of Care (CLIA WAIVED) Rapid Antigen Test offers several key features that make it an excellent choice for COVID-19 testing:
- Rapid results in 20 minutes
- Easy-to-use with minimal training required
- High sensitivity and specificity for accurate detection
- Suitable for various point-of-care settings
- No specialized equipment needed
These features ensure that healthcare providers can perform testing quickly and efficiently, helping to identify and manage COVID-19 cases effectively.
What’s Included in the Phase Scientific INDICAID Point of Care Rapid Antigen Test
The INDICAID Point of Care Rapid Antigen Test kit includes all the necessary components to perform the test accurately and efficiently. The kit is designed for ease of use, ensuring that healthcare providers have everything they need.
- 25 INDICAID test devices
- 25 sample collection swabs
- 25 reagent solution vials
- 1 package insert with detailed instructions
- 1 quality control test set
These items ensure that each test can be performed with precision and reliability, providing healthcare professionals with confidence in the results.
How to Use the Phase Scientific INDICAID Point of Care (CLIA WAIVED) Rapid Antigen Test
Using the INDICAID Point of Care Rapid Antigen Test is straightforward and can be completed in just a few steps:
- Collect the nasal swab sample from the patient.
- Insert the swab into the reagent solution vial and mix.
- Apply the solution to the test device.
- Wait 20 minutes for the results to develop.
- Read the results as indicated on the test device.
These simple steps allow for quick and accurate testing, making it easier to manage and monitor COVID-19 cases.
Where to Buy the Phase Scientific INDICAID Point of Care (CLIA WAIVED) Rapid Antigen Test
The Phase Scientific INDICAID Point of Care Rapid Antigen Test is available for purchase through various authorized distributors. For the most reliable service and competitive pricing, consider ordering from GSE Medical Supplies. GSE Medical Supplies offers fast shipping and excellent customer service to ensure you receive your testing supplies promptly.
Resources
- Indicaid Safety Data Sheet
- Quick Reference Guide
- Frequently Asked Questions – For Patients
- Indicaid Frequently Asked Questions for Healthcare Providers
Frequently Asked Questions
What is the accuracy of the Phase Scientific INDICAID Point of Care Rapid Antigen Test?
The INDICAID Point of Care Rapid Antigen Test has been shown to have high sensitivity and specificity, making it a reliable option for detecting active COVID-19 infections. It is important to follow the instructions carefully to ensure the most accurate results.
How long does it take to get results from the INDICAID Rapid Antigen Test?
Results from the INDICAID Test are available within 20 minutes, making it a fast and efficient option for point-of-care testing. This rapid turnaround is crucial for timely decision-making in clinical settings.
Can the INDICAID Rapid Antigen Test be used for asymptomatic individuals?
Yes, the INDICAID Test can be used to test both symptomatic and asymptomatic individuals. However, it is recommended to confirm negative results with a PCR test, especially in cases of high suspicion of COVID-19 infection.
Who manufactures the Phase Scientific INDICAID Point of Care Rapid Antigen Test?
The INDICAID Point of Care Rapid Antigen Test is manufactured by Phase Scientific International, a biotechnology company focused on developing innovative diagnostic solutions. Their mission is to provide high-quality and accessible testing options to improve global health.
What other products does Phase Scientific offer?
In addition to the INDICAID Rapid Antigen Test, Phase Scientific offers a range of diagnostic products, including PCR tests, antibody tests, and other point-of-care solutions. Their products are designed to meet the diverse needs of healthcare providers and contribute to better patient outcomes.
Why should I buy from GSE Medical Supplies?
GSE Medical Supplies is a trusted distributor of medical products, including the Phase Scientific INDICAID Rapid Antigen Test. When you purchase from GSE Medical Supplies, you benefit from competitive pricing, fast shipping, and exceptional customer service. GSE is committed to providing healthcare providers with the tools they need to deliver the best care possible.
About GSE Medical Supplies
Global Supply Exchange (GSE) Medical Supplies is a leading provider of medical products and diagnostic tools, offering a wide range of supplies to healthcare professionals and organizations. With a commitment to quality, GSE ensures that all products meet the highest standards for reliability and effectiveness. By ordering from GSE, customers benefit from competitive pricing, fast and reliable shipping, and a customer-focused approach that prioritizes their needs. Trust GSE Medical Supplies to deliver the products you need to keep your practice running smoothly and efficiently
FDA Letter of Authorization
INDICAID™ Quick Reference Guide
INDICAID™ Instructions for Use
Patient Fact Sheet
Fact Sheet For Healthcare Providers
Safety Data Sheet INDICAID COVID-19 Rapid Antigen Test
Quality Controls for INDICAID COVID-19 Rapid Antigen Test (IFU)