Description
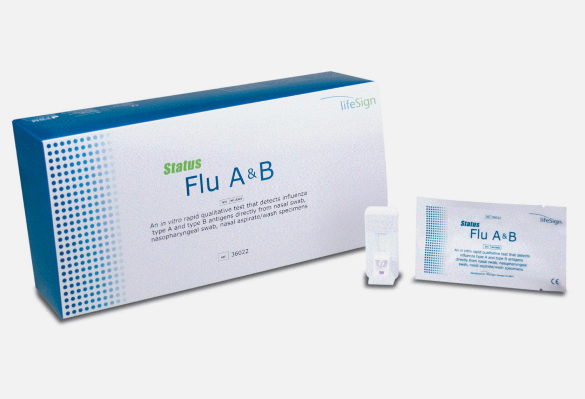
The Status™ Flu A&B Test is a rapid immunoassay designed to detect and differentiate between Influenza A and Influenza B viral antigens in nasopharyngeal specimens. With results in as little as 10 minutes, this CLIA-required test empowers healthcare providers to make fast, evidence-based treatment decisions at the point of care.
Key Features:
-
✅ Detects Both Influenza A and B Viruses
-
✅ CLIA-Moderate Complexity Required
-
✅ Fast Results in 10 Minutes
-
✅ Distinct Results for Flu A & B
-
✅ No Additional Equipment Needed
-
✅ FDA Cleared for Clinical Use
What’s Included:
-
25 Test Cassettes
-
Sterile Nasopharyngeal Swabs
-
Extraction Reagents
-
Positive & Negative Controls
-
Instruction Manual
How It Works:
-
Collect nasopharyngeal specimen using the swab provided.
-
Mix with buffer solution and apply to the test cassette.
-
Wait 10 minutes and visually interpret the result.
-
Read separate lines for Influenza A, Influenza B, and Control.
Who Should Use It:
-
Urgent Care Centers
-
Physician Offices
-
Hospitals & Clinics
-
Flu Testing Sites
-
Mobile Health Units
Benefits for Clinical Settings:
-
Early Detection = Early Treatment: Helps determine antiviral therapy eligibility.
-
Reduced Antibiotic Overuse: Differentiates flu from bacterial infections.
-
Improves Patient Flow: Quick results lead to better care decisions.
-
Supports Outbreak Management: Essential during flu season and community spread.
Compliance & Certifications:
-
✅ CLIA Complexity: Moderate (CLIA Certificate Required)
-
✅ FDA 510(k) Cleared
-
✅ Intended for Professional Use
Technical Specifications:
-
Sample Type: Nasopharyngeal swab
-
Test Time: 10 minutes
-
Storage: Room temperature (15–30°C / 59–86°F)
-
Shelf Life: 24 months from manufacture date
Order with Confidence
Global Supply Exchange provides reliable diagnostic tools for healthcare providers nationwide. With competitive pricing, fast shipping, and responsive customer support, we are your trusted supplier for medical testing solutions.